A research team led by S. Komar Kawatra, a professor of chemical engineering at Michigan Tech University are testing ways to capture carbon emissions from fossil-fueled power plants and turn it into usable products.
Turning carbon emissions into rare earth metals
The Michigan Tech research team is working on a method to convert the CO2 produced by electricity generation into oxalic acid, a naturally-occurring chemical that is used in the technology industry to leach rare earth metals from ore.
Rare earth metals are crucial components in many low-carbon technologies, including electric motors for wind turbines and EVs as well as solar panels. They’re also used in speakers, microphones, and other electronics.
With more and more cities and states pledging to source 100% of their electricity from renewable resources, demand for rare earth metals is also set to grow and we’ll need a lot more of them if states wish to meet these goals.
China currently processes 90% of the world’s rare earth metals. In these unstable times, when the US has slapped tariffs on Chinese-made solar panels and caused the solar industry to enter a slump, that could be an issue. However, the researchers say, if manufacturers could process oxalic acid here in the US, we might be able to profitably extract rare earth metals domestically.
Cost is the biggest challenge to carbon capture adoption
Michigan Tech’s research could do more for the environment than creating a method to domestically-source materials for clean energy technology, much of which is both manufactured and assembled in China and imported as ready-to-go products.
One of the biggest challenges with carbon capture is simply the enormous cost of adding capture equipment to power plants. But by turning the captured CO2, which would otherwise simply be buried underground, into a commercially viable product, power plant owners would be able to offset some of that high installation cost and possibly shift the balance of cost-effectiveness.
Beyond producing commercially-viable products from the emissions, simply reducing operating costs can also affect a carbon capture system’s bottom line.
In their testing, the Michigan Tech researchers are using a novel approach to ‘scrubbing’ power plant emissions to collect all the CO2. Conventional systems use amines, a family of organic solvents that bind to the carbon dioxide and make it easier to collect. However, amines are quite expensive.
Michigan Tech is experimenting with using soda ash, a cheaper alternative, to remove the carbon dioxide from the rest of the emissions. It’s this cost savings that is drawing John Simmons, chairman of Carbontec Energy in North Dakota, to the project. He says amines cost $20,000 a ton, soda carbonates cost just $200 per ton.
Coupling this lower materials cost with the manufacture of a commercially-viable product, could shift the scales towards CCS as financially-viable option.
The DOE is actively funding research into using carbon emissions for products that could permanently absorb the CO2, namely cement, plastics, mineralization, and enhanced oil recovery. The report notes that there’s well over a dozen other potential products as well:
Michigan Tech isn’t the only group looking to turn carbon emissions into usable products. The Congressional Research Service, the research arm of the US Congress, published a report on carbon capturing in August 2018 and noted that the federal government and private companies are gaining interest in Carbon Capture, Utilization, and Sequestration (CCUS).
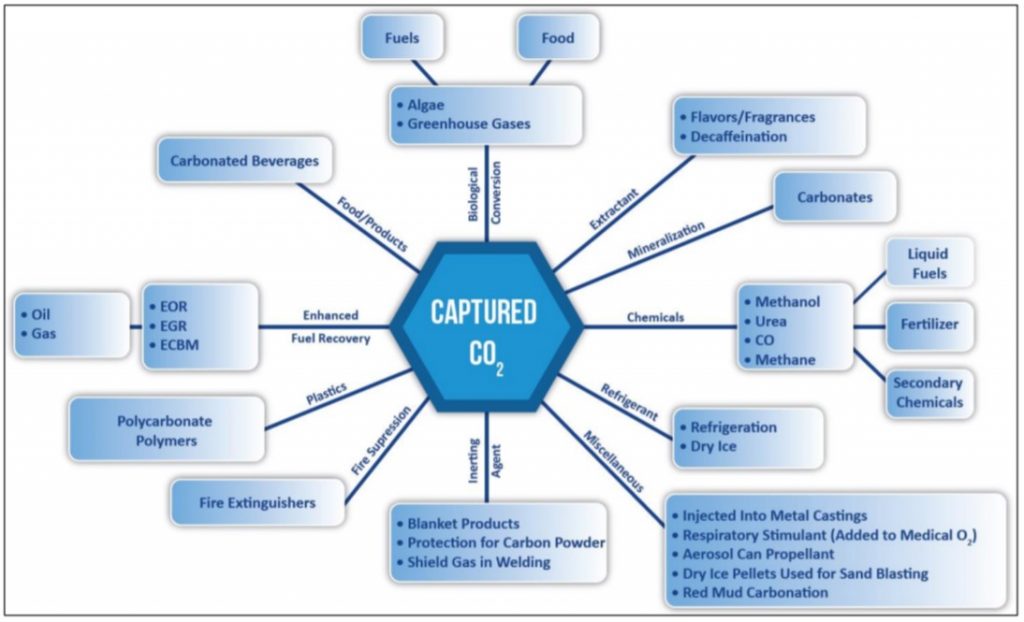
How do you capture carbon emissions?
Capturing carbon emissions, known as Carbon Capture and Sequestration (or Storage, known as CCS either way) is a way to decrease the emissions from coal and natural gas-fired power plants, but still have the flexibility and reliability of a fossil-fueled plant.
It’s seen as way to drastically cut carbon emissions – up to 90% – without actually building any new renewable resources. Some states allow utilities to include CCS in meeting state-wide clean-energy goals. California’s newly-adopted 100% clean energy bill, for example, could possibly allow natural gas-fueled generation to continue if the emissions are captured, though concerns would likely exist around the efficacy of long-term storage and the fact that, even though it’s emitting far less pollution, it’s still powered by fossil fuels.
The coal- and natural-gas fired power plant Petra Nova in Texas is the only large-scale CCS project in the US. The plant started its CCS venture in 2017 and captures 90% of CO2 emissions. This works out to 5,000 tons per day, and 1.5 million tons each year.
After capturing emissions, plant owners must then decide what to do with the CO2. Experts say gas fields, unmineable coal seams, and deep saline reservoirs could also be used for storage, as long as there’s a hard, nonpermeable caprock above, to keep the carbon emissions from escaping into the atmosphere.
The Petra Nova plant transports the CO2 through an 82 mile pipeline to the West Ranch Oil Field, where it’s injected into the oil fields to increase production – a process known as Enhanced Oil Recovery (CO2 EOR). By using the captured emissions for EOR, plant owners are solving two challenges. First, the oil fields are a convenient storage site. Secondly, just like selling Michigan Tech’s product, selling the CO2 helps offset the high costs of installing the carbon capture equipment.
If efforts like Michigan Tech’s research truly finds a way to turn CO2 emissions into usable products – like oxalic acid for processing rare earth metals – we might find that our clean-energy future includes, ironically, fossil-fuels. However, with the public and economy increasingly supporting renewable technologies like solar and wind, not just clean-emissions energy, CCS might just be a stopgap measure until we’re able to move to 100% renewable.