Bismuth – part of the active ingredient in the stomach-settler Pepto-Bismol – might just be the key to making cheap, rechargeable and green batteries that could be used on a big scale to store renewable energy.
Researchers at the University of Southern California, backed by the government’s ARPA-E program, report that additions of bismuth sulfide to iron-air batteries reducing the amount of power lost to hydrolysis from 50 percent to 4 percent. This approximately tenfold increase in efficiency for the batteries, which came on the scene in the 1970s, might make them a player in the all-important quest to find ways to store intermittently produced clean energy.
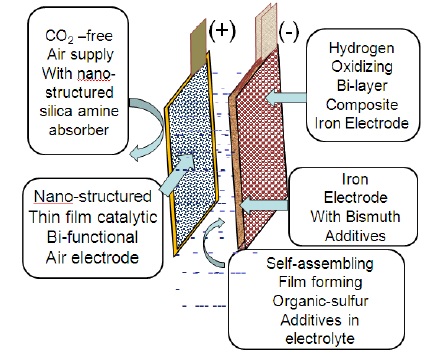
“Iron is cheap and air is free,” Sri Narayan, the USC chemistry professor heading up the research, said in a university release. “It’s the future.”
Iron-air batteries work by using the chemical energy generated by the oxidation of iron plates when exposed to the oxygen in air, but that chemical reaction has heretofore been significantly offset competing hydrogen generation taking place in the battery.
Narayan said tempering the hydrolysis effect could have been accomplished with the addition of lead or mercury on the battery’s iron-based electrode. But using bismuth sulfide – it’s bismuth subsalicylate, or “pink bismuth” that goes into Pepto-Bismol – avoids those toxins.
“A very small amount of bismuth sulfide doesn’t compromise on the promise of an eco-friendly battery that we started with,” he said.
The iron-air rechargeable battery research at USC was funded to the tune of $1,459,324 through the Advance Research Projects Agency-Energy program, overseen by the U.S. Department of Energy.
The Department of Energy’s ARPA-E program has been a big Obama administration priority. Although established in 2007 under President George W. Bush, it didn’t receive any funding until President Obama’s 2009 stimulus provided $400 million.
Then in the current budget ARPA-E got $275 million, half the administration’s request of $550 million [PDF], but an increase over its 2011 funding of $180 million, and much higher than the Republican House bill that sought to cut ARPA-E funding to $100 million.
The work at USC is one of 12 research programs funded by a part of APRA-E called GRIDS, for “Grid-Scale Rampable Intermittent Dispatchable Storage.” The goal is to develop technologies that can be used to store renewable energy for use at any location on the grid at an investment cost less than $100 per kilowatt-hour.
On Thursday this week, the U.S. announced even more money for energy storage: The DOE said 19 projects would receive a total of $43 million through two new ARPA-E programs — Advanced Management and Protection of Energy Storage Devices (AMPED) and Small Business Innovation Research (SBIR). The government-backed R&D “will focus on innovations in battery management and storage to advance electric vehicle technologies, help improve the efficiency and reliability of the electrical grid and provide important energy security benefits to America’s armed forces,” the DOE said.
Batteries are already getting some attention to store power from renewables, but because of the size and expense of current battery technology, these efforts are mainly about smoothing flow and mitigating utility challenges at peak load.
According to the DOE, “If successful, USC’s iron-air battery would represent a low-cost alternative to the best commercial batteries in use today. This technology could be scaled up to provide substantial storage capacity for the use of renewable power within the electric grid.”

While USC noted that Narayan and his team were “working to make the battery store more energy with less material,” the researchers appear extremely confident they had made a big breakthrough with their work. In addition to the bismuthadditives on the battery’s iron-based electrode, the researchers have been restructuring the catalysts at the molecular level on the battery’s air-based electrode, helping the battery resist degradation and increase life span.
“These high-performance electrodes,” they wrote in their Journal of the Electrochemical Society paper, “have broken the once-formidable barrier of low charging efficiencies and unneeded hydrogen evolution in iron-based aqueous alkaline batteries. Thus, both iron-air and nickel iron batteries can now become the basis for low-cost, durable, and efficient large-scale electrical energy storage systems.”