A breakthrough at the UC San Diego School of Engineering could offer an environmentally friendly and less expensive way to produce hydrogen—a carbon-free fuel—by taking advantage of a novel tree-like nanostructure. The vertical branch structure, or “nanotree,” is used to maximize solar energy capture and hydrogen gas output.
“With this structure, we have enhanced, by at least 400,000 times, the surface area for chemical reactions,” said Ke Sun, a Ph.D. student in electrical engineering who led the project.
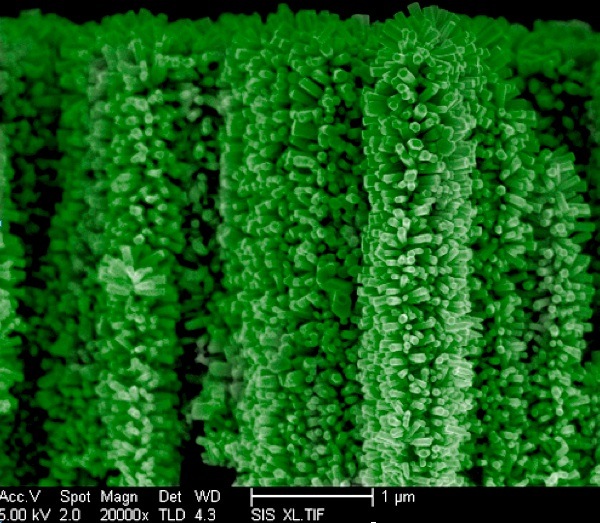
Reporting in the journal Nanoscale, the team said they are building a forest of tiny nanowire trees in order to cleanly capture solar energy in a way that mimics the way trees absorb energy.
Like the famous work by Daniel Nocera, artificial photosynthesis is the inspiration for the UC San Diego Jacobs School of Engineering team working under Deli Wang, professor in the Department of Electrical and Computer Engineering. (We’ve also seen MIT’s Andreas Mershin devise an “electric nanoforest” in the quest for cheap solar power.)
In photosynthesis, as plants absorb sunlight they also collect carbon dioxide (CO2) and water from the atmosphere to create carbohydrates to fuel their own growth.
Wang’s team also hopes to mimic this natural photosynthesis process to capture CO2 from the atmosphere, reducing carbon emissions, and convert it into hydrocarbon fuel.
Most of this promising hydrogen research is focused on substituting clean energy with no greenhouse gas byproduct for today’s fossil-fueled hydrogen splitting. Wang’s “3D branched nanowire array” uses a process for separating water into oxygen and hydrogen called photoelectrochemical water-splitting that uses sunlight.
Most research in the field of carbon-friendly solar hydrogen splitting is also bent on finding a substitute for today’s expensive platinum catalyst, which keeps hydrogen fuel cells prohibitively expensive. The options range from Duke University’s idea of producing hydrogen from rooftop solar collectors—glass vacuum tubes filled with water and methanol and catalytic nanoparticles—to Nocera’s cheap cobalt and nickel catalysts.
But in addition to using the future’s plentiful sunlight—and cheap and abundant silicon and zinc oxide nanowires—Wang’s breakthrough also involves a nanoscale structural change in how the sunlight is actually absorbed. This structure is key in improving the efficiency of the process.

The trees’ vertical structure and branches are keys to capturing the maximum amount of solar energy.
That’s because the vertical structure of trees grabs and adsorbs light while flat surfaces simply reflect it, Wang said, adding that it is also similar to retinal photoreceptor cells in the human eye. In images of Earth from space, light reflects off flat surfaces such as the ocean or deserts, while forests appear darker.
The vertical branch structure also maximizes hydrogen gas output, said Sun. For example, on the flat, wide surface of a pot of boiling water, bubbles must become large to come to the surface. In the nanotree structure, very small gas bubbles of hydrogen can be extracted much faster, enhancing the surface area by as much as 400,000 times.
Once cheap enough, widespread uses of hydrogen fuel cells would make storing energy much cheaper, in buildings, on the grid, and in vehicles—ranging from cheaper fuel-cell vehicles at military installations on the gas-dependent island of Hawaii—to possibly the most adorable single occupancy vehicle ever designed.